
We explore these types in more detail below: Whole virus vaccineĪlso known as an “inactivated” or “weakened” virus vaccine, this type contains dead or inactivated forms of the virus.
VACCINE TIMELINE PROFESSIONAL
It is essential to receive the vaccine from a licensed healthcare professional and follow every instruction, including getting a second dose. This helps them monitor the impact of the vaccine and do ongoing work to ensure public safety. The CDC encourage people to use a smartphone-based health checker called V-safe to inform the authorities about any side effects. The side effects may be worse after the second dose of the vaccine because the body’s immune response will be intensified. In the short term, a person who has had a COVID-19 vaccine may experience flu-like symptoms and other side effects, including:
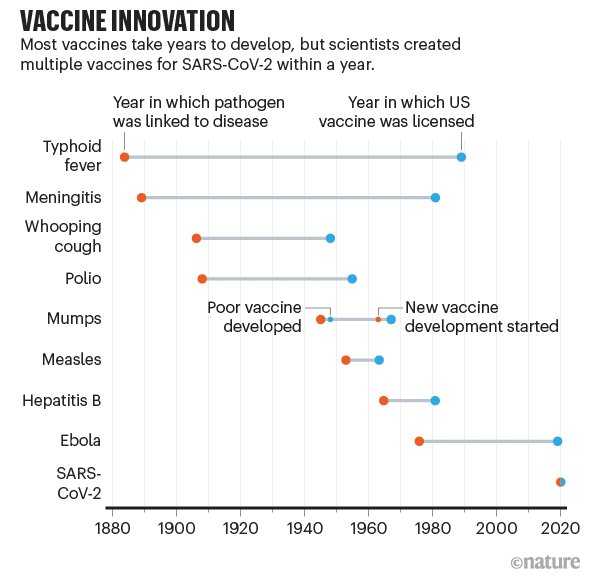
The key is to balance the potential risks of getting a vaccine that has undergone extensive testing with the known dangers of developing COVID-19. The last stage, phase 3, includes tens of thousands of participants.Īt first, the specific long-term effects of any new medical treatment, including a vaccine, are unknown. Vaccine trials involve ever-larger numbers of people. In the U.S., the FDA gives this approval, and the Centers for Disease Control and Prevention (CDC) also work to ensure public safety. Both were developed by companies based in the U.S.Ī person can keep up to date with the latest vaccine developments in the country using the Regulatory Affairs Professionals Society’s COVID-19 vaccine tracker.Ī vaccine needs to pass through several stages of trials before the manufacturer can apply for approval from a country’s health authority.

Meanwhile, the Novavax vaccine is currently undergoing phase 3 trials, as is Janssen’s COVID-19 vaccine. Covaxin, developed by Bharat Biotech, in India.Coronavac, developed by Sinovac, in China.The Oxford AstraZeneca vaccine, in the United Kingdom.Other vaccines that have approval for use in various countries include: The results indicated that the vaccine was 94% effective. In a phase 3 trial, 30,000 volunteers received either a placebo or two doses of the vaccine, 28 days apart. The Moderna vaccine, developed in Cambridge, MA, received approval for emergency use in the U.S.

The results showed that the vaccine was 95% effective at protecting against COVID-19.
VACCINE TIMELINE TRIAL
In a phase 3 trial involving more than 43,000 people, around half received a placebo and half received two doses of the vaccine, 21 days apart. The Pfizer-BioNTech vaccine, developed in Germany, received FDA approval in the form of an emergency use authorization on December 11, 2020. The last stage, phase 3, involves tens of thousands of participants.Īt the time of writing, two vaccines have FDA approval for use in the U.S.: In the United States, vaccines need approval from the Food and Drug Administration (FDA).įirst, they need to pass through three phases of tests to prove that they are safe and effective. Different vaccines are now available in various countries.


 0 kommentar(er)
0 kommentar(er)
